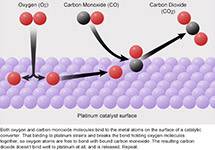
Catalytic Chemistry
Catalysis is an acceleration or retardation of the rate of a chemical reaction, brought about by the addition of a substance (the catalyst ) to the reaction medium. Most chemical reactions in industry and biology are catalytic and play a role at some stage of the processing of about 80% of the goods manufactured in the, yet catalysis is a neglected subject in chemical education. Only very small amount of catalyst is needed to generate copious amounts of product. This is desirable, as many catalysts that are used industrially are very expensive. By choosing the appropriate catalyst, a particular reaction can be made to occur to the extent of practically excluding another. Many important applications of catalysis are based on selectivity of this kind. a catalyst does not affect the position of equilibrium of a chemical reaction; it affects only the rate at which equilibrium is attained.
- Auto Catalysts
- Enzymes and Biocatalysts
- Tandem Catalysts
Related Conference of Catalytic Chemistry
13th International Conference and Exhibition on Chromatography and Analytical Techniques
5th World Congress on Plasma Chemistry and Plasma Processing
6th International Conference on Petrochemistry and Natural Gas
Catalytic Chemistry Conference Speakers
Recommended Sessions
- Analytical Chemistry
- Biopolymers
- Catalytic Chemistry
- Chemical Plants Development
- Chemistry of Transition Elements
- Desalination
- Electrochemistry
- Food, Beverage & Tobacco
- Geochemistry
- Geosciences
- Green Water Treatment
- Hazardous Waste Management
- Industrial Chemistry
- Industrial Photo Chemistry
- Industrial Water Supply
- Infection Risks Due to Contaminated Water
- Material Science & Chemical Metallurgy
- Medicinal Chemistry
- Organic Acids, Amines, Esters and Phenols
- Organic Chemistry
- Paper, Pulp & Fibre
- Petro-Chemistry
- Pharmaceutical Chemistry
- Pharmaceuticals & Nutraceuticals
- Plasma Chemistry
- Pyrolysis
- Raw Water / Potable Water Preparation
- Removal of Pollutants from Water Using Different Types of Nanomaterials
- Ultrapure Water Production
- Waste Water Treatment
Related Journals
Are you interested in
- 2D Materials and van der Waals Heterostructures - Materials Chemistry-2026 (France)
- Advanced Ceramics for High-Tech Applications - Materials Chemistry-2026 (France)
- Advanced Forensic Chemistry - Forensic Chemistry 2026 (UAE)
- Advanced Functional Materials for Next-Generation Technologies - Materials Chemistry-2026 (France)
- Advanced Medicinal Chemistry - Chemistry Meeting-2026 (France)
- Advanced Organic & Inorganic Chemistry - Chemistry Meeting-2026 (France)
- Advanced Organic & Inorganic Chemistry - Euro Chemistry 2026 (Spain)
- Advancements in Analytical Techniques - Analytical Techniques 2026 (France)
- Advancements in Mass Spectrometry - Analytical Techniques 2026 (France)
- Advances in Chromatography Instrumentations - Chromatography Techniques-2026 (France)
- Advances in Chromatography-HPLC Instrumentations - Advanced Chromatography 2026 (Italy)
- Advances in Separation Techniques - Analytical Techniques 2026 (France)
- Agricultural Chemistry - Chemistry Meeting-2026 (France)
- AI and Machine Learning in Drug Design - PHARMACOLOGY CONGRESS 2026 (UAE)
- Analytical & Bio-analytical Applications of Chromatography - Advanced Chromatography 2026 (Italy)
- Analytical & Bio-analytical Applications of Chromatography - Chromatography Techniques-2026 (France)
- Analytical Challenges in Personalized Medicine - Analytical Techniques 2026 (France)
- Analytical Chemistry - Chemistry Meeting-2026 (France)
- Analytical Chemistry - Euro Chemistry 2026 (Spain)
- Analytical Chemistry and Methodology - Analytical Techniques 2026 (France)
- Analytical Chemistry in the Energy Sector - Analytical Techniques 2026 (France)
- Applications of mass spectrometry - Analytical Techniques 2026 (France)
- Applied Chemistry - Chemistry Meeting-2026 (France)
- Applied Chemistry - Euro Chemistry 2026 (Spain)
- Automation in Analytical Labs - Chromatography 2026 (Italy)
- Battery Materials and Next-Generation Energy Storage - Materials Chemistry-2026 (France)
- Bio-Based Hydrogels & Soft Biomaterials - biopolymerscongress-2026 (France)
- Bioanalytical Chromatography - Chromatography 2026 (Italy)
- Bioanalytical Methodology - Analytical Techniques 2026 (France)
- Biochemical Applications of Chromatography - Chromatography Techniques-2026 (France)
- Biochemical Applications of Chromatography-HPLC - Advanced Chromatography 2026 (Italy)
- Biochemical Markers in Forensics - Forensic Chemistry 2026 (UAE)
- Biochemistry - Chemistry Meeting-2026 (France)
- Biochemistry - Euro Chemistry 2026 (Spain)
- Biochemistry - Analytical Techniques 2026 (France)
- Biodegradable Polymers for Medical Devices - biopolymerscongress-2026 (France)
- Bioinspired and Biomimetic Material Systems - Materials Chemistry-2026 (France)
- Biologics, Biosimilars, and Monoclonal Antibodies - PHARMACOLOGY CONGRESS 2026 (UAE)
- Biomaterials for Neural Repair & Interfaces - biopolymerscongress-2026 (France)
- Biopolymer Applications in Wound Care - biopolymerscongress-2026 (France)
- Biopolymer Innovations in 3D/4D Bioprinting - biopolymerscongress-2026 (France)
- Biopolymer Nanofibers & Advanced Scaffolds - biopolymerscongress-2026 (France)
- Biopolymers and Biomaterials - Analytical Techniques 2026 (France)
- Capillary Electrophoresis - Chromatography 2026 (Italy)
- Cardiovascular Pharmacology and Therapeutics - PHARMACOLOGY CONGRESS 2026 (UAE)
- Chemical Engineering - Chemistry Meeting-2026 (France)
- Chemical Engineering - Euro Chemistry 2026 (Spain)
- Chemistry in Clinical Research - Chemistry Meeting-2026 (France)
- Chemistry in Clinical Research - Euro Chemistry 2026 (Spain)
- Chip Based Chromatography Separations - Advanced Chromatography 2026 (Italy)
- Chip Based Chromatography Separations - Chromatography Techniques-2026 (France)
- Chiral Separation Methods - Chromatography 2026 (Italy)
- Chromatographic Method Validation - Chromatography 2026 (Italy)
- Chromatography - Analytical Techniques 2026 (France)
- Chromatography & Mass Spectrometry - Advanced Chromatography 2026 (Italy)
- Chromatography as Separation Techniques - Chromatography Techniques-2026 (France)
- Chromatography in Bio-Medical Research - Chromatography Techniques-2026 (France)
- Chromatography in Food Science Technology - Advanced Chromatography 2026 (Italy)
- Chromatography in Food Science Technology - Chromatography Techniques-2026 (France)
- Chromatography in Pharmacy & Pharmaceutical - Advanced Chromatography 2026 (Italy)
- Chromatography in Pharmacy & Pharmaceutical - Chromatography Techniques-2026 (France)
- Chromatography-HPLC as Separation Techniques - Advanced Chromatography 2026 (Italy)
- Chromatography-HPLC in Bio-Medical Research - Advanced Chromatography 2026 (Italy)
- Chromatography-Mass Spectroscopy Analysis - Chromatography Techniques-2026 (France)
- Clinical and Diagnostic Bioanalysis - Analytical Techniques 2026 (France)
- Clinical Diagnostics Applications - Chromatography 2026 (Italy)
- Clinical Diagnostics Equipment and Kits - Analytical Techniques 2026 (France)
- Clinical Trials and Regulatory Affairs in Drug Development - PHARMACOLOGY CONGRESS 2026 (UAE)
- Column Technology Developments - Chromatography 2026 (Italy)
- Computational Chemistry - Chemistry Meeting-2026 (France)
- Computational Chemistry - Euro Chemistry 2026 (Spain)
- Conductive Polymers for Wearable Sensors - biopolymerscongress-2026 (France)
- Corrosion Science and Protective Material Coatings - Materials Chemistry-2026 (France)
- Crime Scene Investigation and Reconstruction - Forensic Chemistry 2026 (UAE)
- Digital and Cyber Forensics - Forensic Chemistry 2026 (UAE)
- Drug Discovery and Development Strategies - PHARMACOLOGY CONGRESS 2026 (UAE)
- Eco-Friendly Bioplastics & Green Alternatives - biopolymerscongress-2026 (France)
- Edible Films & Smart Food Packaging - biopolymerscongress-2026 (France)
- Electrochemistry - Chemistry Meeting-2026 (France)
- Electrochemistry - Euro Chemistry 2026 (Spain)
- Electrophoresis - Analytical Techniques 2026 (France)
- Emerging Research and Innovations - Forensic Chemistry 2026 (UAE)
- Emerging Trends in Immunopharmacology - PHARMACOLOGY CONGRESS 2026 (UAE)
- Environmental Analytical Chemistry - Analytical Techniques 2026 (France)
- Environmental Forensics - Forensic Chemistry 2026 (UAE)
- Environmental Sample Analysis - Chromatography 2026 (Italy)
- Food & Beverage Testing - Chromatography 2026 (Italy)
- Forensic Anthropology and Facial Reconstruction - Forensic Chemistry 2026 (UAE)
- Forensic Anthropology and Odontology - Forensic Chemistry 2026 (UAE)
- Forensic Botany and Entomology - Forensic Chemistry 2026 (UAE)
- Forensic Chromatographic Tools - Chromatography 2026 (Italy)
- Forensic Engineering and Accident Reconstruction - Forensic Chemistry 2026 (UAE)
- Forensic Genomics and Epigenetics - Forensic Chemistry 2026 (UAE)
- Forensic Instrumentation and Technology - Forensic Chemistry 2026 (UAE)
- Forensic Linguistics - Forensic Chemistry 2026 (UAE)
- Forensic Psychology and Behavioral Science - Forensic Chemistry 2026 (UAE)
- Forensic Serology - Forensic Chemistry 2026 (UAE)
- Forensic Toxicology - Forensic Chemistry 2026 (UAE)
- Future Trends in Biomaterial Safety & Standards - biopolymerscongress-2026 (France)
- Gas Chromatography Advances - Chromatography 2026 (Italy)
- Green and Sustainable Chemistry - Chemistry Meeting-2026 (France)
- Green and Sustainable Chemistry - Euro Chemistry 2026 (Spain)
- Green Chromatography Solutions - Chromatography 2026 (Italy)
- High Efficiency & High Resolution Techniques - Advanced Chromatography 2026 (Italy)
- High Efficiency & High Resolution Techniques - Chromatography Techniques-2026 (France)
- High-Throughput Screening - Chromatography 2026 (Italy)
- High-Throughput Screening and Lead Optimization - PHARMACOLOGY CONGRESS 2026 (UAE)
- HPLC Fingerprinting in Bioinformatics & Computational Biology - Advanced Chromatography 2026 (Italy)
- HPLC Fingerprinting in Bioinformatics & Computational Biology - Chromatography Techniques-2026 (France)
- Hybrid Materials and Organic–Inorganic Interfaces - Materials Chemistry-2026 (France)
- Hyphenated Chromatography Methods - Advanced Chromatography 2026 (Italy)
- Hyphenated Chromatography Methods - Chromatography Techniques-2026 (France)
- Hyphenated Techniques (GC-MS, LC-MS) - Chromatography 2026 (Italy)
- Industrial Chemistry - Chemistry Meeting-2026 (France)
- Innovation in Catalytic Materials and Surface Chemistry - Materials Chemistry-2026 (France)
- Innovation in Food Analysis & Testing - Analytical Techniques 2026 (France)
- International Collaboration in Forensic Science - Forensic Chemistry 2026 (UAE)
- Legal and Ethical Aspects - Forensic Chemistry 2026 (UAE)
- Liquid Chromatography Techniques - Chromatography 2026 (Italy)
- Major Chromatographic Techniques - Advanced Chromatography 2026 (Italy)
- Major Chromatographic Techniques - Chromatography Techniques-2026 (France)
- Marine & Algae-Derived Biopolymers - biopolymerscongress-2026 (France)
- Market Growth of Chromatography - Chromatography Techniques-2026 (France)
- Market Growth of Chromatography-HPLC - Advanced Chromatography 2026 (Italy)
- Materials Chemistry - Chemistry Meeting-2026 (France)
- Materials Chemistry - Euro Chemistry 2026 (Spain)
- Materials for Biomedical and Regenerative Applications - Materials Chemistry-2026 (France)
- Materials for Extreme Environments and High-Performance Applications - Materials Chemistry-2026 (France)
- Materials for Sustainable and Clean Energy Conversion - Materials Chemistry-2026 (France)
- Materials Informatics and AI-Driven Material Discovery - Materials Chemistry-2026 (France)
- Measuring surfactants - Advanced Chromatography 2026 (Italy)
- Metabolomics & Proteomics Analysis - Chromatography 2026 (Italy)
- Metamaterials and Programmable Material Platforms - Materials Chemistry-2026 (France)
- Method Development & Validation - Advanced Chromatography 2026 (Italy)
- Method Development & Validation - Chromatography Techniques-2026 (France)
- Microscopy - Analytical Techniques 2026 (France)
- Modern Forensic Biochemistry - Forensic Chemistry 2026 (UAE)
- Nanobiomaterials for Targeted Therapies - biopolymerscongress-2026 (France)
- Nanochromatography Innovations - Chromatography 2026 (Italy)
- Nanomaterials and Nanotechnology in Analysis - Analytical Techniques 2026 (France)
- Nanostructured Materials and Molecular Engineering - Materials Chemistry-2026 (France)
- Nanotechnology-Based Drug Delivery Systems - PHARMACOLOGY CONGRESS 2026 (UAE)
- Natural Product Chemistry - Chemistry Meeting-2026 (France)
- Natural Product Chemistry - Euro Chemistry 2026 (Spain)
- Natural Products and Phytopharmaceuticals - PHARMACOLOGY CONGRESS 2026 (UAE)
- Neuropharmacology and CNS Drug Research - PHARMACOLOGY CONGRESS 2026 (UAE)
- New Instrumentation and Equipment - Analytical Techniques 2026 (France)
- NMR and Analysis of small Organic Molecules - Analytical Techniques 2026 (France)
- Novel Approaches to analytical and Bioanalytical method - Analytical Techniques 2026 (France)
- Oncology Drug Discovery and Targeted Therapies - PHARMACOLOGY CONGRESS 2026 (UAE)
- Pharmaceutical Analytics - Analytical Techniques 2026 (France)
- Pharmaceutical and Drug Forensics - Forensic Chemistry 2026 (UAE)
- Pharmaceutical Chemistry - Chemistry Meeting-2026 (France)
- Pharmaceutical chemistry - Euro Chemistry 2026 (Spain)
- Pharmaceutical Quality Control - Chromatography 2026 (Italy)
- Pharmacogenomics and Personalized Medicine - PHARMACOLOGY CONGRESS 2026 (UAE)
- Pharmacokinetics, Pharmacodynamics, and ADME Studies - PHARMACOLOGY CONGRESS 2026 (UAE)
- Photonic and Optoelectronic Materials Advancements - Materials Chemistry-2026 (France)
- Physical Chemistry - Chemistry Meeting-2026 (France)
- Physical Chemistry - Euro Chemistry 2026 (Spain)
- Polymer Chemistry - Chemistry Meeting-2026 (France)
- Polymer Chemistry - Euro Chemistry 2026 (Spain)
- Polymer Science and Material Science - Analytical Techniques 2026 (France)
- Protein and Peptide Analysis - Chromatography 2026 (Italy)
- Proteomics and its applications - Analytical Techniques 2026 (France)
- Quantum Materials and Ultrafast Phenomena - Materials Chemistry-2026 (France)
- Real-Time Data Analytics in Chromatography - Chromatography 2026 (Italy)
- Recent Advances in Chromatography - Chromatography Techniques-2026 (France)
- Recent Advances in Chromatography-HPLC - Advanced Chromatography 2026 (Italy)
- Recyclable, Lightweight, and High-Strength Composite Materials - Materials Chemistry-2026 (France)
- Smart Biopolymers for Regenerative Healing - biopolymerscongress-2026 (France)
- Smart Polymers and Soft Matter Innovations - Materials Chemistry-2026 (France)
- Stimuli-Responsive Smart Materials - biopolymerscongress-2026 (France)
- Supramolecular Chemistry - Chemistry Meeting-2026 (France)
- Sustainable Biocomposites for Industry - biopolymerscongress-2026 (France)
- Sustainable Materials Chemistry for a Circular Economy - Materials Chemistry-2026 (France)
- Synthesis and Characterization of Novel Materials - Materials Chemistry-2026 (France)
- Theoretical Chemistry - Chemistry Meeting-2026 (France)
- Theoretical Chemistry - Euro Chemistry 2026 (Spain)
- Toxicology, Safety Assessment, and Risk Management - PHARMACOLOGY CONGRESS 2026 (UAE)
- Translational Pharmacology and Preclinical Models - PHARMACOLOGY CONGRESS 2026 (UAE)